- Fig 1 (im Cache).
- Kritik an dieser Vereinfachung: C.M. Eggleston et al., "Surface complexation of sulfate by hematite surfaces: FTIR and STM observations", Abstract, chached, full text.rft, Fig.1, Fig.2, Fig.3.
- Tiberg C; Sjöstedt C; Persson I; Gustafsson JP, "Phosphate effects on copper(II) and lead(II) sorption to ferrihydrite" - author's version, Geochimica et Cosmochimica Acta, Volume 120, 1 November 2013, Pages 140 - 157 (im Cache - surface complexes).
- Tiberg C; Gustafsson JP, "Phosphate effects on cadmium(II) sorption to ferrihydrite", Journal of Colloid and Interface Science 471 (2016) 103 - 111 (im Cache - surface complexes), authors' version with Suppl. Mat. 2016 (im Cache)
While the 1:1 aqueous carbonato species (NpO2CO3-) was found to become predominant in the circumneutral pH range, it is most likely that this species is sorbed onto the gibbsite surface as a ternary inner sphere surface complex where the NpO2+ moiety is directly coordinated to the functional groups of the gibbsite's surface. These findings are corroborated by results obtained from EXAFS spectroscopy providing further evidence for a bidentate coordination of the Np(V) ion on amorphous Al(OH)3. The identification of the Np(V) surface species on gibbsite constitutes a basic finding for a comprehensive description of the dissemination of neptunium in groundwater systems.
"Key recommendations for the process of TSM [thermodynamic sorption models] development include:
- definition of modelling objectives,
- identification of major decision points,
- a clear decision-making rationale with reference to experimental or theoretical evidence,
- utilisation of a suitable consultative and iterative model development process,
- testing to the maximum practicable extent, and<
- thorough documentation of key decisions."
"The basic principles of the transport and retention processes should be largely independent of the particular scientific discipline, but interdisciplinary interactions are not necessarily as well-established as they ought to be.
... spectroscopy is not always successful in elucidating mechanisms, since even at the spectroscopic level obviously the interpretation of spectra is required and various mechanisms or combinations of processes might explain the experimental features.
... Experimental work within FUNMIG ...
[FUNMIG = EURATOM 6th EC Framework Program Integrated Project "Fundamental Processes of Radionuclide Migration", 2005 - 2009, one overall objective of the project: improve the knowledge-base for assessment of the long-term safety of a nuclear waste repository in the three host-rock types presently considered in Europe]
... has shown that experimental results on apparently simple surfaces with simple techniques may strongly differ. The gibbsite case shows that acid-base properties of well-defined colloids are not trivial. For example, within FUNMIG, isoelectric points obtained for gibbsite were substantially higher than the bulk of existing values. An explanation for the high isoelectric points has been proposed here, but there is still no explanation for the reason why Rosenqvist el al. (2002) measured charging curves 10 times steeper than the ones obtained by many others and also within FUNMIG on particles made the same way and even studied with the same experimental stetup as the one used by Rosenqvist et al (2002). The repercussion of this lack of understanding on the uptake data for radionuclides (and in particular on the modeling via the acid-base model) is obvious.
... For the Fe- and Al-bearing minerals it became clear that the occurrence of certain surface species should never be inferred from previous observations made on similar or even nominally identical sorbents. ... Different predominance of various crystal planes with various surface sites may even make this conclusion applicable to nominally identical solids."
... The complex reactivity of dissolved Al3+ at the plagioclase-water interface severely complicated the development of a surface complexation model, emphasizing the need for additional research in this area. Our study highlights the importance of molecular-level studies to understand surface reactions and uncover unknown processes, which may have significant impact on the transport of (radiotoxic) contaminants in the geosphere.
"... Although, right now, the overall picture appears to be clear from a generic point of view (i.e. concerning the trends), clearly, in a quantitative sense, huge differences occur for nominally identical systems and only such a comprehensive study will allow to proof the current phenomenological picture and allow the next step to be taken to understand the fine details of the complex clay-electrolyte solution interfaces."
"... One has to keep in mind that the titrations (that also have been used in the cited theoretical work for comparison) may be misleading. Titrations yield relative changes, whereas the models used to extract pK values in surface complexation models will require absolute values (of charge of protons adsorbed or whatever quantity is used to fit surface complexation parameters). These absolute values are typically not available for clays. ..."
"... Overall, a comprehensive study involving a multitude of experimental and theoretical approaches, with close collaboration to exactly mimic the experimental surfaces in the calculations, would be highly beneficial. This conclusion has also been drawn for seemingly simpler systems such as metal oxide surfaces.
Although the advances in the detailed understanding and the possibilities with modern experimental and computational techniques are breathtaking, much can be expected to surprise us in the future when taking an even closer look at mineral-electrolyte solution interfaces.
- "Generell hängt die Zuverlässigkeit und Genauigkeit geochemischer Rechnungen entscheidend von der Qualität der verfügbaren Daten ab, und
- strenge Qualitätssicherungsmaßnahmen werden gefordert.
- Die Verwendung nachweislich qualitätsgesicherter und konsistenter Daten ist daher unabdingbar.
- Die möglichen Auswirkungen von Datenunsicherheiten sind zu diskutieren.
- Gegebenenfalls sind Resultate geochemischer Rechnungen durch gezielte Experimente zu überprüfen und zu validieren."
Source of picture: Altman et al, 2015
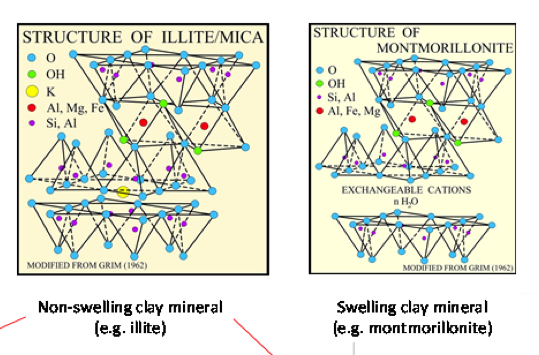
The main features of compacted bentonite and clayrocks influencing RN migration can be summarized as follows (cf. Figure 1):
- The porosity of highly compacted bentonites and clayrocks is dominated by pore spaces in contact with the different categories of clay mineral surfaces: edges, external basal, interlayer (smectite-type only). The pore volume dimensions and the proportioning of total pore space between the external and interlayer fractions vary as a function of bentonite dry density.
- The composition of the pore solution is determined by local (pseudo)equilibrium involving dissolved components, certain mineral phases and clay surface functional groups; the dissolved concentrations of certain components (e.g. Cl-, Na+) may be determined by externally imposed conditions. The dissolved speciation of diffusing trace elements is at equilibrium with the pore solution.
- Clay external and interlayer surfaces possess characteristic densities of permanent negative charge compensated in solution by equivalent amounts of positive charge in the form of cationic species. Cations compensating charge located on external basal surfaces are distributed between two populations: that in direct contact and as dissolved species present in the adjacent solution.
- Electrostatic attraction and repulsion respectively of cations and anions by external and internal surfaces determine the porosities accessible to different species; cations and neutral species can access most if not all of the total connected porosity, while anions are generally assumed to be entirely excluded from the interlayer volumes of highly compacted clay masses and more or less excluded (depending on the ionic strength) from the solution volume immediately adjacent to external basal surfaces.
- The edges of the TOT layers of smectite and illite clay minerals possess functional groups capable of sorbing cationic Me species.
The essential characteristics of Thermodynamic Sorption Models (TSM) for cation sorption on illite and smectite type minerals of interest for coupling with diffusion behaviour can be summarized by the following points:
A variety of mass action laws for both basal and edge site species have been proposed representing different hypotheses regarding the stoichiometry, molecular scale'geometry' and the contribution of electrostatic forces to the stability of each type of surface site - cation species association. They reflect different compromises across the spectrum of TSM modelling objectives extending from efficient representation of sorption datasets for evaluating and defending Kd values used in performance assessment to capturing as closely as possible available knowledge regarding surface species fine structure and bond energetics. The main model differences of interest in the context of coupling surface speciation to diffusion are (i) whether or not the totality of the surface charge associated with external basal surfaces is considered to be compensated by S:Me species, and (ii) the stoichiometry and stability (also reversibility) of edge site surface complexes.
- Sorption of alkali element cations (Cs(I), K(I), Rb(I), ...) and alkaline earth divalent cations (Sr(II), Ca(II), ...) is determined by interactions with structural negative charge localisations on basal and interlayer surfaces. Three site types have been identified for alkali cation sorption on illite: abundant but low energy 'planar' sites (PS), lower abundance but higher energy 'type II' sites (T2) and low abundance, high specifity 'frayed-edge sites (FES). PS and T2 sites should be present in montmorrillonite basal and interface surfaces. Surface complexation on edge sites (see below) may also contribute slightly to alkaline earth cation fixation. (Bradbury and Baeyens, 2000 and 2005a, Brower et al., 1983)
- Sorption of transition metal, lanthanide and actinide cations involves at least two surface complexation type sites located on the edges; a low concentration, high energy 'weak'site (WS). In addition, at least one cation exchange site (PS), possibly also 'T2', is needed at low pH.