 |
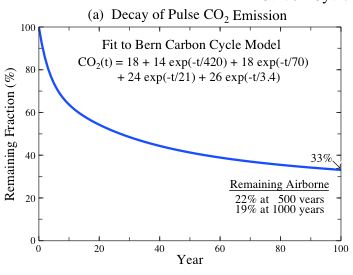
Fig. 9a: Decay of a small pulse of CO2 added to today's atmosphere, based on analytic approximation to the Bern carbon cycle model (Joos F et al., An efficient and accurate representation of complex oceanc and biospheric models of anthropogenic carbon uptake, Tellus, 48B, 397-417, 1996; Shine et al., Alternatives to the global warming potential for comparing climate impacts of emissions of greenhouse gases, Clim. Change, 68, 281-302, 2005, see equation given in figure).
In this approximation of the carbon cycle,
|
Integration over the period 1750 - 2005 of the product of this equation and fossil fuel emissions yields a present airborne fossil fuel CO2 amount of approx. 80 ppm (Kharecha PA, Hansen JE, 2007).
The observed atmospheric CO2 increase is approx. 100 ppm, the difference presumably due to the net consequence of deforestation and biospheric uptake not incorporated in the carbon cycle model, and, in part, imprecision of the carbon cycle model. This calculation provides a check on the reasonableness of the carbon cycle model approximation of [the given equation] ...
Archer D, Fate of fossil-fuel CO2 in geologic time, J. Geophys. Res., 110, C09S05, doi:10.1029/2004JC002625, 2005
Source: Fig. 9a and page 2302 in Hansen J. et al., Dangerous human-made interference with climate: a GISS model-study, Atmospheric Chemistry and Physics, Vol. 7 (2007), pp. 2287-2312 (in cache).
Kharecha PA, Hansen JE, Implications of "peak oil" for atmospheric CO2 and climate, 2007 arXiv:0704.2782v3 [physics.ao-ph] (in cache).
