Source: C. Tiberg, J.P. Gustafsson, "Phosphate effects on cadmium(II) sorption to ferrihydrite", Journal of Colloid and Interface Science 471 (2016) 103–111 (in cache)
Phosphate effects on cadmium(II) sorption to ferrihydrite
Charlotta Tiberg a,⇑, Jon Petter Gustafsson a,b
a Department of Soil and Environment, Swedish University of Agricultural Sciences (SLU), Box 7014, SE-750 07 Uppsala, Sweden
b Division of Land and Water Resources Engineering, KTH Royal Institute of Technology, SE-100 44 Stockholm, Sweden
Journal of Colloid and Interface Science 471 (2016) 103–111

Source: C. Tiberg, J.P. Gustafsson, "Phosphate effects on cadmium(II) sorption to ferrihydrite", Journal of Colloid and Interface Science 471 (2016) 103–111 (in cache)
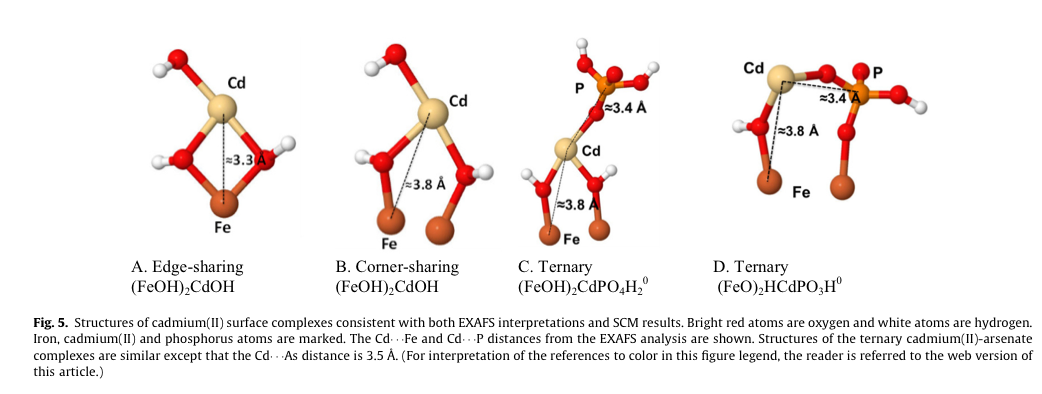
Conclusions
Phosphate greatly enhanced cadmium(II) sorption to ferrihydrite. The increased sorption could not be explained by electrostatic interactions alone. A ternary ferrihydrite-cadmium(II)phosphate surface complex had to be considered. This study confirmed our hypotheses that ... it should be possible to identify the surface complexes formed by EXAFS spectroscopy and implement these complexes in a surface complexation model (SCM). Previous studies have shown increased sorption of cadmium(II) to iron (hydr)oxides in the presence of phosphate, but only to goethite. Our study is also the first to identify ternary iron (hydr)oxide-cadmium(II)-phosphate surface complexes by EXAFS spectroscopy and to successfully implement such complexes in a SCM, the CD-MUSIC model. In contrast to earlier EXAFS measurements of cadmium(II) sorbed to goethite in presence of phosphate we identified a Cd - P distance indicative of a ternary complex at about 3.4 Å by EXAFS. The existence of a ternary complex was supported by an earlier ATR-FTIR analysis of a cadmium(II)-phosphate-hematite system.
Verson: 17.3.2016
Adress of this page
Home
Joachim Gruber